This page is historical material reflecting the Feedback Loop Blog as it existed on
August 19, 2014. This page is no longer updated and links to external websites
and some internal pages may not work.
August 19, 2014
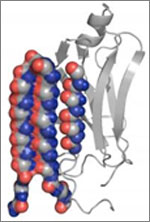
A molecule targets the intermediary structure of a protein involved in disease-associated amyloid fibrils. Credit: University of Washington.
Alzheimer’s disease, type 2 diabetes and many other illnesses are linked to the buildup of proteins whose structures have changed into shapes that enable the formation of cell-entangling threads called amyloid fibrils. About 10 years ago, researchers led by Valerie Daggett of the University of Washington used computer simulations to suggest that such proteins, on their way to creating fibrils, form an intermediary structure called an alpha sheet that’s even more toxic to cells than fibrils. Now Daggett’s team has experimentally investigated this possibility. The scientists made alpha sheet molecules expected to bind to amyloid-forming proteins in the computationally predicted intermediate state. When they tested the molecules on two amyloid disease-related proteins, they observed a substantial reduction in fibril formation. The work is still very preliminary, but it highlights a potential new avenue for treating a range of amyloid-related diseases.
This work also was funded by NIH’s National Institute of Allergy and Infectious Diseases.
Learn more:
University of Washington News Release  Daggett Lab
Daggett Lab 
Monster Mash: Protein Folding Gone Wrong Article from
Inside Life Science